
Enjoy A Lucrative Salary And High Personal Fulfillment As You Ensure That Safe And Effective Therapies Become Available To The General Public
Introducing The Regulatory Affairs Career Track By Cheeky Scientist

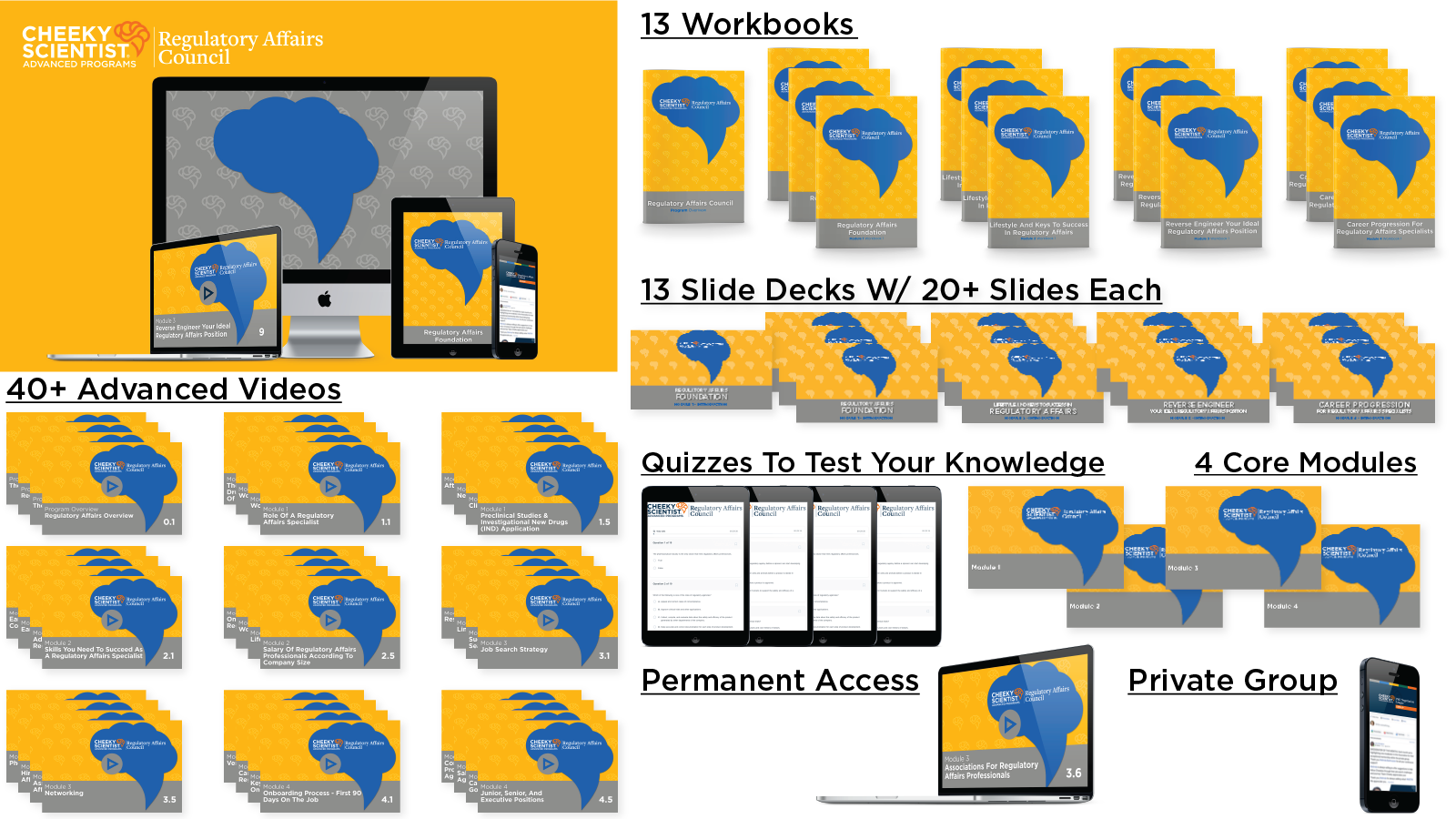
Regulatory Affairs Career Track
Take Action Now To Become A Diamond Member And Get Hired As A Regulatory Affairs Specialist!
Here’s What You Get Immediate Access To When You Become A Cheeky Scientist Association Diamond Member…
Reverse-Engineer Your Ideal Management Consultant Position With A Targeted Mentoring Program

“I enjoyed reviewing the Regulatory Affairs Career Track because it describes the regulatory affairs career track accurately. Sometimes regulatory affairs is perceived as a rigid career path, but the Regulatory Affairs Career Track does a good job at portraying the versatility of the field and how PhDs can contribute to innovate, develop, produce, and market a variety of products while ensuring safety and effectiveness. The program also provides its members with a network of regulatory affairs professionals and PhDs interested in regulatory affairs, which will further support their transition from academia to industry. I especially liked how the material represents the subfields of the Regulatory Affairs Career Track, how they are different, and how they overlap. The program also covers knowledge that PhDs need to succeed as regulatory affairs specialists and how you developed them during grad school. The Regulatory Affairs Career Track discusses the differences between regulatory affairs specialists working in government agencies vs companies with an emphasis on the application process and career progression path. This will help members set the right career and networking strategy.”

“The Regulatory Affairs Career Track covers the regulatory affairs field, job hiring process, and career track, weighing the options in industry and the government along with differences between different countries. So, PhDs wanting to transition into regulatory affairs could benefit from this program no matter their location or position of interest. The program provides PhDs with an end-to-end overview of the lifestyle and hiring process of the regulatory affairs field, what you should consider when onboarding as a regulatory affairs specialist, and a discussion of career options and how your knowledge can transfer to other roles.”
The Regulatory Affairs Career Track Is Designed for PhDs Who Want To…
- Transition into a growing field that values professionals capable of understanding current and changing regulations and best practices.
- Leverage attention to detail and critical thinking to advise biopharma and biotech companies on the best way to develop and keep products in the market.
- Gain clarity on how to break into the regulatory affairs field.
- Secure a position where you’re sought by recruiters offering high salaries.
- Learn how to convince managers and different departments that following your recommendations will benefit their companies.
- Get familiar with the terminology and approaches commonly used by regulatory affairs specialists so you can impress future employers.
- Know how to mediate between regulatory bodies and private companies to benefit the general public.
- Apply your analytical and scientific skills to solve real-life problems.
- Learn what the regulatory affairs hiring process looks like and what knowledge you should highlight.
The Demand For Regulatory Affairs Specialists is High, And You Don’t Need Legal or Clinical Experience To Get Hired. You Just Need Your PhD…
Your knowledge—or lack thereof—will play a key role in your regulatory affairs job search.
You may have a PhD, and you may understand that breaking into the field doesn’t begin with resumes.
But you need to understand current regulations and be comfortable with a specific language to convince employers that you can help them develop their projects without creating compliance issues.
Even though demand for this position is high, you’ll need to showcase specific skills to edge out your competitors and land your first role in this industry.
Growth Of The Regulatory Affairs Market In The U.S
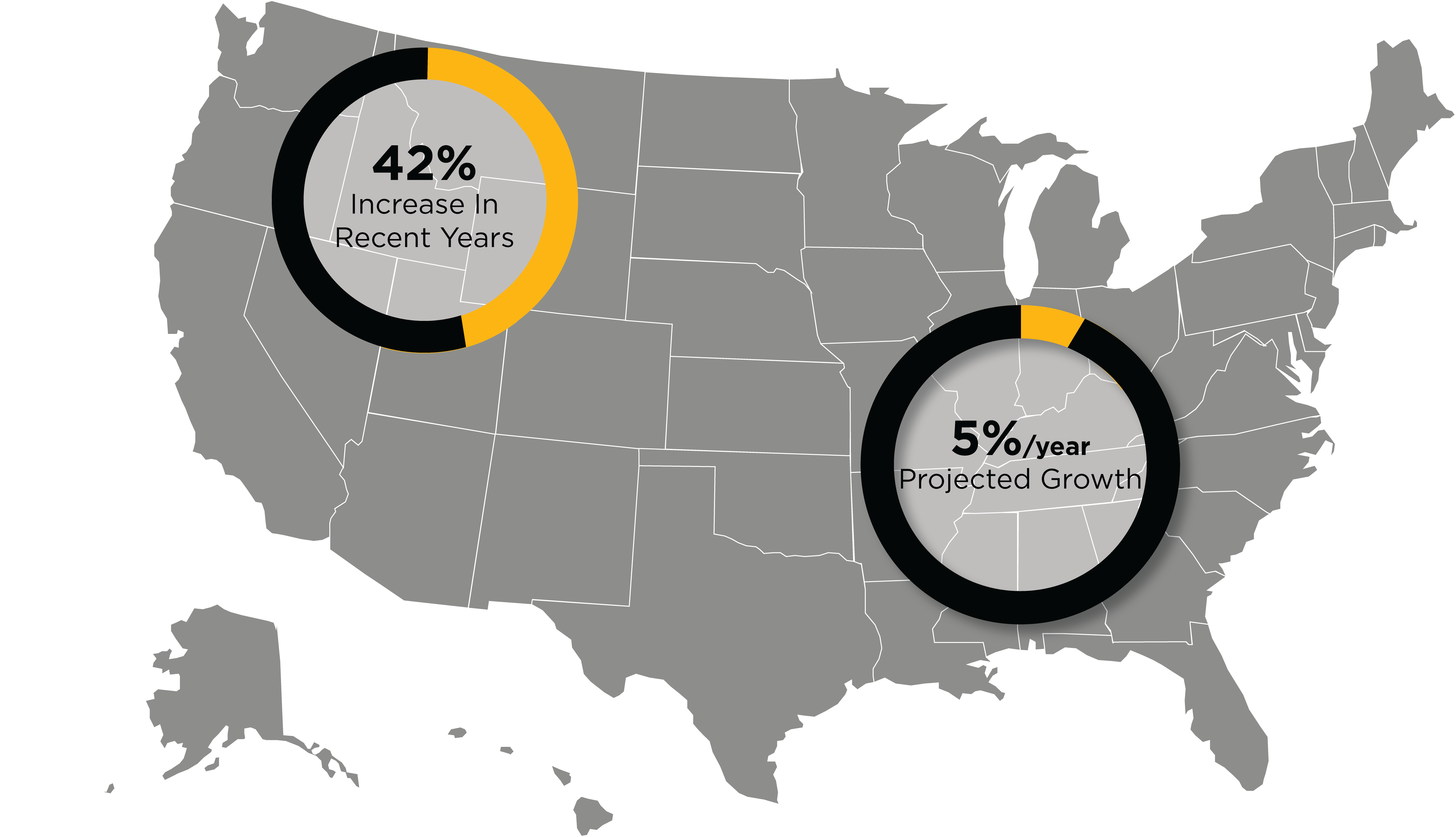
- The need for Regulatory Affairs Specialists in the U.S is projected to grow by around 5% per year in the following decade (U.S Bureau of Labor Statistics).
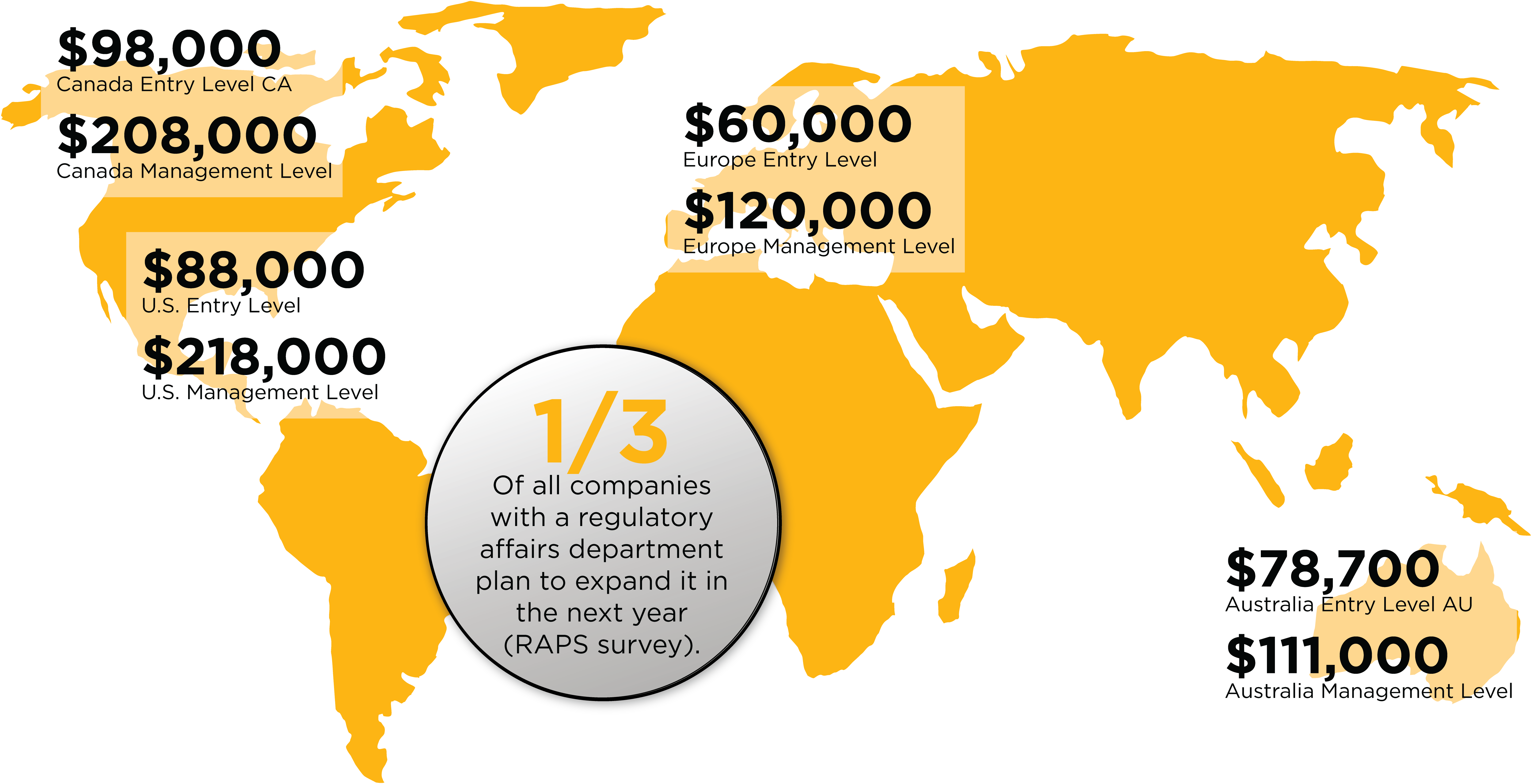
- The average entry level salary for a regulatory affairs specialist is $88,000. This goes up to $281,000 for a management position (RAPS survey).
- ⅓ of all companies with a regulatory affairs department plan to expand it in the next year (RAPS survey).
- With the increase in regulations imposed by regulatory bodies, the demand for professionals with regulatory expertise will increase (Northeastern University).
- The U.S. regulatory market experienced a 42% increase in job listings in recent years (Massachusetts Biotechnology Education Foundation).

“The Regulatory Affairs Career Track materials offer a well-planned blueprint for PhDs wishing to break into regulatory affairs roles. The documents lay out the why, the where, and the how of setting up a successful job search strategy, as well as possible vertical and lateral career moves you can make once you gain regulatory affairs experience.”

“The structure of the Regulatory Affairs Career Track – Overview, Introduction, Lifestyle, Hiring Process, Career Progression – is easy to follow and should be beneficial for any PhD considering a career in regulatory affairs. The program provides enough depth of content in each module to allow overall understanding of the Regulatory Affairs profession, professionals, recruitment steps and professional growth. The details provided about the hiring process should accelerate the transition of members regardless of their location or desired position.”

“The Regulatory Affairs Career Track gives a bird's-eye view of the regulatory affairs field. The program explains very well the role of a Regulatory Affairs Specialist, as well as the steps of the drug-approval process. The Regulatory Affairs Career Track also covers the horizontal moves PhDs can pursue once they gain regulatory experience. Knowing that you have several opportunities to move within different areas is really helpful. Since I have experience in regulatory affairs, I understand how knowing the terminology and acronyms is critical in getting a job in this area and I really like that this program offers guidelines are on how to get familiar with regulatory terminology.”

If You’re A PhD Looking To Transition Into Industry, See If You Answer YES To These Questions…
- Do you want to play an active role in making the best possible medicines, equipment, and treatments for patients in need?
- Do you want to learn how regulations in different countries impact which pharmaceutical products reach the market?
- Do you want to mediate between private sponsors and regulatory bodies to reach outcomes beneficial for both?
- Do you want to use your academic background to help managers understand the importance of following best practices in all departments of a company?
- Do you want to work with the government, making sure that beneficial products reach the market in your country?
- Do you want to have a say on the regulations that govern the pharmaceutical and biotech industries and play a role in interpreting these regulations?
Regulatory Affairs Specialists Form An Exclusive Group
Regulatory affairs specialists earn fantastic pay, enjoy excellent work-life balance, and have a say in how different companies operate and comply with regulations.
But these are privileges that they’ve earned.
Members of this group speak their own language.
They know how to keep up to date with regulations from different agencies and how to advise business management according to them.
Regulatory affairs specialists mediate the most difficult and high-stakes conversations in the biopharma and biotech industries and play an active role in which products reach the market.
If you want to increase your chances of becoming a regulatory affairs specialist, you can’t apply for this position blindly.
You need detailed information on your future position. During a job search, you can’t afford to show you don’t know which are the most relevant regulatory agencies to the companies you want to work with. You can’t risk revealing that you don’t know the implications of missing a deadline or sending an incorrect regulatory document.
To avoid blunders like these, you’ll want to thoroughly understand what it means to be a regulatory affairs specialist and what a typical day for one looks like.

The key skills employers in private companies and governmental bodies are looking for in a regulatory affairs specialist…
Regulatory affairs specialists have a skill set that empowers them to meet the unique challenges of their position. Your future employer is looking for these qualities. You’ll want an insider’s perspective on how to demonstrate that you have the abilities needed to excel in your regulatory affairs position.
Get familiar with specific terms and acronyms of the regulatory affairs world. Regulatory affairs professionals use different acronyms for important documents, processes, and agencies. To be successful, you’ll want to show that you recognize these terms, understand what they mean, and know why they are important.
Once You’re A Regulatory Affairs Specialist, You’ll Experience Professional Respect, Opportunities, And Much Responsibility
When you imagine a job where you use your PhD education, you probably think of several key qualities: professional respect, responsibility, and personal autonomy…
Those are some of the reasons you’re attracted to a regulatory affairs position, but here’s something that’s just as important:
These opportunities aren’t confined to playing an active role in shaping the pharmaceutical market.
Becoming a regulatory affairs specialist can also provide an incredible amount of financial opportunities.
If you’re currently conducting postdoc work, this can seem too good to be true…
After all, you’re used to earning a salary far beneath your education.
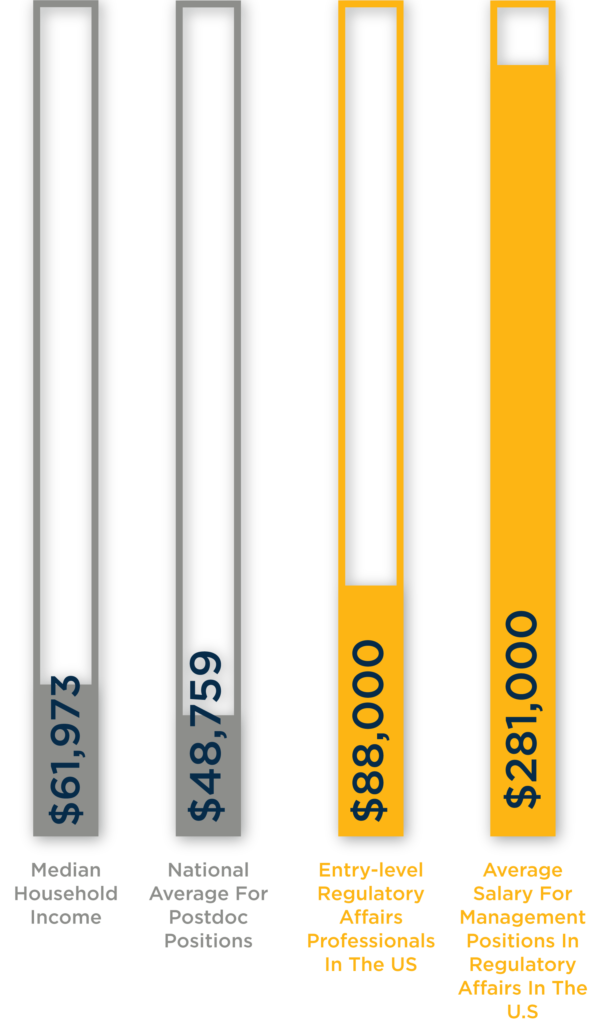
The median household income is $61,937 (U.S. Census Bureau).
But even though you have a PhD, you probably earn well below that figure.
The national average for postdoc positions is $48.759 (glassdoor).
But when you become a regulatory affairs professional, you will be among the most sought-after professionals in the pharma and biotech industries, with financial opportunities you’ve never experienced before.
The average annual salary for an entry-level regulatory affairs specialist in the U.S is $88,000 (RAPS survey).
And don’t forget that this is for entry level positions.
Regulatory affairs managers professionals in the U.S earn $281,000 on average.
Additionally, positions in the regulatory affairs field offer some of the best work-life balance among PhD-level positions. This is especially true if you work for governmental agencies.
You will also have abundant opportunities to branch out into other roles if you wish, such as medical writer, medical affairs professional, clinical research associate, and quality control manager, among others.
Once You’re A Regulatory Affairs Specialist, You’ll Experience A Stunning Amount of Opportunities, And Professional Respect
3 Important Reasons To Join The Association’s Diamond Program
1. You Want To Play An Active Role In Deciding Which Healthcare Products Become Available To The Public.
Regulatory affairs specialists are the ones in charge of making sure that safe and effective products and treatment reach the market in as little time as possible. Thus, they significantly influence the healthcare landscape.
2. You Want The Option Of Working For The Private Or Public Sector.
Regulatory affairs professionals can work for private companies, advising them on how to develop their products and keep them in the market after approval. They also work for public regulatory bodies, helping create the regulations that govern different industries and reviewing applications to determine which products are allowed to go to the market.
3. You Want To Advise Different Departments About The Best Way To Develop Products While Complying With Regulations and Best Practices.
Regulatory affairs professionals are in charge of understanding and keeping up to date with the regulations and best practices that govern their industries and countries. They also need to be able to explain to different professionals why it is important to comply with regulations.
The Regulatory Affairs Career Track Can Pay For Itself In 3 Days Or Less.
A Position In Regulatory Affairs Has You Earning Over $1,500 Per Week And Over $200 Per Day…
If joining the Association’s Diamond Program puts you in the field even one week faster, the investment will have been worth it.
After that one payment, you’ll have access to all Cheeky Scientist mentoring materials, and once you secure your position, you can share your insights with other PhDs like you.
The Regulatory Affairs Career Track Includes Ongoing Validation To Ensure You Are Always Up-To-Date As A Regulatory Affairs Specialist
You will receive an official letter of recommendation from our Regulatory Affairs Career Track board upon completion of the program along with lifetime access to our annual validation program.
The Regulatory Affairs Career Track was created and is maintained by a board of regulatory affairs professionals.
As such, each module includes a board-certified exam, and once you pass all the exams, you will receive a ribbon of completion from our board.
The Regulatory Affairs Career Track Mission
In the Regulatory Affairs Career Track, we’re committed to supporting you on your journey to landing a top regulatory affairs position by providing you with advanced, actionable strategies for success.
To do just that, we’ve provided extensive mentoring from current regulatory affairs professionals already in industry, leading-edge regulatory affairs-targeted resume templates, and guidance by an intimate group of practicing regulatory affairs professionals and other PhDs aspiring to this role.
We understand why you want to break into this industry.
You want to translate your scientific knowledge into safe and effective products.
You want to help managers create strategies for drug development that comply with regulations.
You desire to impact the healthcare industry, work with different departments of a company, and serve as a mediator between the private and public sectors.
But here’s the reality…
Just because this field is growing doesn’t mean it isn’t competitive.
Frankly, you’re going to need more than a cursory knowledge of the regulatory affairs field to make the best impression with your future employer.
It’s our goal to help you do just that.
The information we’re providing — from videos to resumes — is laser-targeted for regulatory affairs positions and is exclusively for PhDs.
There are nuances to the regulatory affairs field that you will find nowhere else.
The Regulatory Affairs Career Track was built by practicing regulatory affairs professionals who are familiar with the role’s nuances. They can provide you with tools and advice on how best to secure a position.
As a regulatory affairs specialist, your employer wants to know that you understand the importance of different regulations and keeping up to date with them, understand the goals of the company, and can serve as a mediator to ensure that the best therapies reach the market and comply with regulations.
You don’t need to pay thousands of dollars to understand what your employer is looking for.
In the Regulatory Affairs Career Track, we provide extensive mentoring and personalized support. And our materials are specifically designed to help you showcase both your natural talents and your PhD expertise so you attract the attention of leading companies and regulatory bodies.
As A PhD, You Can Transition Into A Role In Regulatory Affairs
You earned your PhD with careful thinking and hard work.
Achieving a regulatory affairs position is no different.
You have the work ethic to make this happen.
You simply need to develop an extensive knowledge of the regulatory affairs positions.
We’re here to decrease your learning curve.
And our goal is the same as yours: securing that regulatory affairs job.
We’ve done it.
We’ve helped other PhDs do it.
And you can do it too.
We’re inviting you to join the Association’s Diamond Program.
Now is the time to close the gap between where you are and where you want to be.
The Regulatory Affairs Career Track Is A Private Group Of Practicing Regulatory Affairs Professionals And PhDs Like You
Our exclusive group is private and provides a number of regulatory affairs mentors, ensuring we can provide personalized guidance.
The mentorship is hyper-focused and the private group support prepares you by filling in your knowledge gaps about regulatory affairs positions and the hiring process.
In the private group, top regulatory affairs professionals currently working in industry are committed to providing a high level of service within this intimate community.
This means you’ll enjoy a high ratio of mentors to members.
You’ll appreciate how the Regulatory Affairs Career Track is designed to provide personalized guidance and swift feedback.

Imagine Walking Into Your First Regulatory Affairs Interview, But You Don’t Know…
- What DMF stands for or why this is relevant in the regulatory field. This causes you to panic when you hear how often the term is used by the hiring manager during your interview.
- The role of regulatory affairs professionals working in the public and private sector at each step of drug development and after the drug hits the market, causing you to make statements that reveal you have little knowledge of the field.
- What questions to ask to demonstrate your knowledge and understanding of the role you’re applying for, which means other—more prepared—candidates leave a better impression with the company and edge you out of the job.
- The terms and vocabulary used during the on-site visit, which makes it difficult to articulate the value of your academic background and show that you would easily transition into the regulatory affairs industry.
- Information about your day-to-day responsibilities, what a regulatory affairs team looks like, and which documents you will be in charge of preparing. This causes you to ask questions during the interview that reveal you have only the most basic information on what your role will entail and what you’ll be expected to accomplish.
The Regulatory Affairs Career Track Guarantees You Will Get Hired Into A Regulatory Affairs Role No Matter Your PhD Background…
The Regulatory Affairs Career Track can translate your field of study into an industry position that puts you on the front lines of drug development working with leading products that have the potential to save lives.
Your PhD has given you the academic training that’s critical for securing these jobs.
But the Regulatory Affairs Career Track can help you prove that fact to employers.
We’ll give you information that’s uniquely relevant to the regulatory affairs positions – not medical affairs, medical writing, or any other field.
The result is intensive, concentrated mentoring that shows you how to integrate your academic background, transferable skills, and knowledge of the regulatory affairs field into behaviors that will help you land a position.
When You Join the Association’s Diamond Program, You’re Joining A Program Designed To Build The Knowledge That Has Helped Other PhDs Land Their Regulatory Affairs Jobs
The Regulatory Affairs Career Track was built on tried and true principles that other – current – regulatory affairs professionals used to land their positions.
The program is run by regulatory affairs professionals who’ve landed industry positions and can mentor you on the knowledge you’ll need to leverage to improve your chances of being hired.
On top of that, the day-to-day activities you’ll learn about in the Regulatory Affairs Career Track are those that other PhDs just like you used to land their first regulatory affairs position to ensure that top-quality, safe, and effective medical products reach the market.
Now that these regulatory affairs professionals have used their experience with the Regulatory Affairs Career Track to successfully transition into industry, they can look back on what helped them secure their regulatory affairs job.
The Private Group Provides Targeted Support

Joining the Association’s Diamond Program lets you tap into targeted support from the regulatory affairs professionals who oversee the program and former students who’ve now landed regulatory affairs positions.
In the privacy of the online group, you’ll be able to ask questions about your resume, gain insights into interview experiences from transitioned regulatory affairs professionals, and interact with like-minded PhDs. That means you can engage in conversations on questions, topics, and challenges relevant to the regulatory affairs field.
This group is also exclusive. Only Cheeky Scientist members can see and respond to the messages in the private group.
The exclusivity of the group allows you to freely share ideas with fellow PhDs, request referrals from other regulatory affairs professionals, and get the advice you need when you encounter challenges.
You’ll get immediate access to a top global network of regulatory affairs professionals and PhDs who want to break into the field once you join…
Here’s the Targeted Mentoring You’ll Receive From The Regulatory Affairs Career Track...

Module #1
Regulatory Affairs Foundations
You know you want to be a regulatory affairs specialist, but you aren’t completely sure of all the responsibilities and expectations that come with the role. This module covers…
- What regulatory affairs is and what the role of a regulatory affairs specialist encompasses. You’ll get insights on the importance of the regulatory affairs field, the responsibilities of the regulatory affairs department, and the difference between working in regulatory affairs for a private company and a regulatory agency.
- The Process Of Getting A Drug Approved. This module shows in detail the steps of the drug development process and how regulatory affairs professionals influence each of these steps. The Module also discusses the role of regulatory affairs professionals once a drug hits the market and presents some of the most relevant regulatory bodies.
- The branches of the regulatory affairs field. This module explores the different career branches that the regulatory affairs field offers and the most important skills for success in each of them.
- Who Hires Regulatory Affairs Specialists. This module gives an overview of the different companies that hire regulatory affairs professionals and what are the differences and similarities between them.

Module #2
Lifestyle and Keys to Success in Regulatory Affairs
You want to know what being a regulatory affairs specialist looks like from day to day and the keys to success in this position. This module covers…
- Earning potential and lifestyle. This module presents the salary expectations of regulatory affairs professionals working around the globe. It also explores the overall trends in the field and the lifestyle of regulatory affairs professionals working in the private and public sector.
- What a day in the life of a regulatory affairs specialist looks like. This module explores the main activities that regulatory affairs specialists must perform on a day to day basis. It also discusses what is the best approach to perform each of those tasks efficiently.
- The most relevant skills of a regulatory affairs specialist. This module presents the most sought-after skills for regulatory affairs specialists, explaining how you have acquired most of them during your PhD and how to leverage them during the hiring process.
- Relevant terminology for regulatory affairs professionals. Half of the preparation for getting into regulatory affairs is knowing the acronyms and getting familiar with the terms. This module presents some of the most common terms used in the regulatory affairs field and their relevance in an industry setting.

Module #3
Reverse Engineer Your Ideal Regulatory Affairs Position
When it comes to securing a job offer as a regulatory affairs specialist, you can significantly improve your chances with the right techniques. This module covers….
- Some key strategies to build a network that will help you transition to the regulatory affairs field. You’ll also gain critical insights into how to build a LinkedIn profile and resume that make you look like an experienced regulatory affairs specialist.
- Associations For Regulatory Affairs Professionals. Being active in an association for regulatory affairs professionals can open doors for you and help you advance your career. This module presents some of the most relevant associations and what they offer to their members.
- What the regulatory affairs hiring process looks like, from phone screen to on-site interview. You’ll also learn what critical questions you can expect during on-site interviews, as well as some questions you can ask to decide if the position is right for you.
- The hiring process of regulatory agencies. Applying to regulatory affairs positions in the public sector is different than applying to the same positions in private companies. This module explains the differences between both types of hiring processes and gives insights into what you should know if you want to succeed in each of them.

Module #4
Career Progression For Regulatory Affairs Specialist
Now that you have secured a position as a regulatory affairs specialist, what next steps should you take to continue growing professionally and personally? This module covers…
- What to do during the first 90 days on the job. This module explores how you should behave during the first few months to guarantee that you create meaningful relationships, are respected by your team, and yield long-term results.
- What career progression looks like for regulatory affairs specialists – the most common vertical moves and how long it generally takes to climb the career ladder.
- Career progression in governmental agencies. This module explores what career progression might look like for you if you decide to work for government agencies. It also discusses the possibility of moving from a private to a public setting and vice versa.
- What your future may hold as a regulatory affairs specialist. Understand the different career paths you can take once you have regulatory affairs experience. This module will help you understand the potential horizontal moves you can make and how they relate to your regulatory affairs and PhD experience.
As Soon As You Join The Association’s Diamond Program, You Will Also Get Immediate Access To The Following…
- A complete guide that provides practical information on the regulatory affairs specialist role and responsibilities, important industry topics, company culture, and more. You’ll enter the job search knowing exactly what you’re looking for and the expectations of your future employer.
- The Regulatory Affairs Career Track describes what you’ll experience in a typical week – from managing timelines to communicating with managers and regulatory bodies. Instead of entering your interview trying to hide what you don’t know, you’ll be prepared to ask important questions so you can determine if you’d accept a job offer from the company.
- Discover what kind of position would best suit your academic background and personal interests and get important information on how to gauge a company’s regulatory culture. Insider tips will help you make savvy decisions as you vie for regulatory affairs job openings.
- Understand the make-or-break skills essential for regulatory affairs specialists so you know how to effectively position yourself. Discover how to translate your natural attention to detail and critical thinking into strategies to position your company while complying with regulations.
- Detailed information on specific regulatory projects and documentation, so you know how to talk to professionals in private companies and regulatory agencies. You’ll understand the challenges that are at the heart of every drug development. With the Regulatory Affairs Career Track, you’ll gain knowledge that shows a potential employer that you’ve done your homework and you’re prepared for a successful transition into industry.
- Understand the role of regulatory affairs specialists in different settings, how to interact with different areas of the company, how to negotiate on behalf of your employer and the general public, and what you need to know about regulatory strategies for different products.
- Gain important insight on the phases of drug development, your expected role in each phase, and the steps it takes to bring a product from ideation to market. You’ll fully understand your role in approving new products and keeping them on the market.
- Discover how to translate your knowledge of the regulatory affairs field into questions that will impress your future company. The mentorship provides important advice to help you showcase your knowledge and passion for the field.
- Access to the private online group: an exclusive circle of regulatory affairs professionals and fellow PhDs where you can communicate about important topics, request referrals, and ask for the input of other regulatory affairs specialists. Only Cheeky Scientist members are allowed access to this group.
- Gain guidance from practicing regulatory affairs professionals on your upcoming interview, your newly created resume, or your recent job offer. They’re in the field and can provide advice that’s targeted for your specific needs and questions.
- Enjoy meaningful conversations with other PhDs in the group. Discuss the latest development in your job search, your current challenges, and more. Learn from PhDs who’ve made the transition and ask for referrals.
- Leverage the private group to find other regulatory affairs specialists or PhDs you can connect with. Find members by their name, location, educational background, or other characteristics to aid your job search.
- Comprehensive mentoring that takes you from understanding the regulatory affairs field to securing your first job. After laying a thorough foundation for your position, the Regulatory Affairs Career Track will cover everything from networking to how to practice for your job interviews so you’re prepared for the hiring process.
- Over the course of four modules, you’ll enjoy information-packed workbooks, numerous videos from regulatory affairs professionals, and exams to help you cement the concepts covered. This mentoring is a crash course on what you need to know to improve your chances of securing a position.
- Discover effective techniques for successfully pinpointing the best companies, targeting your resume for regulatory affairs positions, conducting informational interviews, and more. The Regulatory Affairs Career Track will show you how to tailor your job search so that you secure an offer for a regulatory affairs position.
- Discover the career options available to regulatory affairs specialists. With the Regulatory Affairs Career Track, you’ll be able to look ahead to the future opportunities your new position may create.
- What you need to know about communication as a regulatory affairs specialist, no matter your level of extraversion. The mentorship will underscore the communication techniques that will make a positive impression on your future company and will uniquely qualify you for the role.
- Gain critical insights into the skills needed to successfully transition from your PhD student or postdoc work to life as a regulatory affairs specialist. This mentoring will show you how to showcase the skills that will get you hired.
- Discover what hiring managers are looking for during interviews. With the Regulatory Affairs Career Track, you’ll get important tips for how to behave so you distinguish yourself from other candidates.
- Extensive resources that help you learn the nuances of the regulatory affairs field in-depth.
- The Regulatory Affairs Career Track provides instant access to extensive video mentoring from regulatory affairs professionals. You’ll also gain workbooks, video transcripts, and resume templates for regulatory affairs positions.
- The end of each module provides an exam to solidify the concepts you’ve learned. The Regulatory Affairs Career Track will also provide monthly webinars on important topics relevant to your job search in regulatory affairs.
The Career Track Is NOT For You If...

You Don’t Like To Follow Rules
You like to break boundaries and innovate at any cost. You feel like rules and regulations obstruct your creative process and feel trapped when you have to follow them.
Regulatory affairs professionals are in charge of keeping up to date with regulations and best practices, as well as explaining to different sectors in a company the importance of maintaining compliance.
If you feel that rules were made to break them and they serve no clear purpose, then the Regulatory Affairs Career Track is not for you.
The Regulatory Affairs Career Track is for PhDs who understand that complying with regulations, best practices, and recommendations is the surest way to attain beneficial results for everyone.

You Only Want To Stay In The Lab
More than likely, you went to school with this person.
This person enjoyed hands-on science and was more than happy to spend the majority of each day at the bench with his or her recent experiment as company.
Becoming a regulatory affairs specialist is about understanding the strategies behind drug development instead of developing drugs yourself and it’s about interacting with people at different levels of the company and those working in regulatory agencies and building extensive relationships with them.
If you love scientific breakthroughs but dislike the idea of meeting new people, socializing with important personalities, or strategizing with different teams in your company, then the Regulatory Affairs Career Track isn’t for you.
The Regulatory Affairs Career Track is designed for PhDs who enjoy both science and human interaction.

You Don’t Care For Documentation
You like to do your experiments or build your models, but you don’t like to keep an organized record of what you do or craft documents and reports to convey your progress to others.
Regulatory affairs professionals are in charge of keeping company records organized and preparing regulatory documentation that can be up to a thousand pages long.
If you like to make things happen, but don’t enjoy keeping records, staying organized, or writing documents. Then, the Regulatory Affairs Career Track is not for you.
The Regulatory Affairs Career Track is designed for people who want to tackle extensive documents and keep records of all the processes of the company. They know that keeping those records is a way of shielding the company from legal issues in the future.
Get Access To The Regulatory Affairs Mentoring Program & Network
If you’re determined to be a regulatory affairs specialist and you’ve chosen the Regulatory Affairs Career Track to make that happen, we’d like to welcome you.
As practicing regulatory affairs professionals who oversee the Regulatory Affairs Career Track at the Cheeky Scientist Association, we know firsthand the challenges you face, the questions you have, and the information you’ll need to succeed.
We’re ready to empower you to make the transition, and we hope to meet you in the private group soon.
To your success,
The regulatory affairs professionals at the Cheeky Scientist Association
Regulatory Affairs Career Track Board Members

Rebba Boswell-Casteel, PhD
Evidence Evaluator Manager At Smith & Nephew

Chad Iverson, PhD
Analytical Chemist At Health Canada

Yaping Moshier, PhD, sMBA
Regulatory Affairs Coordinator At University Of Colorado

Dignesh Shah, PhD
Associate Regulatory Affairs Specialist At Prespectum Ltd.

Airama Albisa, PhD
Regulatory Affairs Officer At Farmhispania

Alick Tan, PhD
Regulatory Affairs Specialist II At MicroVention-Terumo

Hoda Erjaee, PhD
Clinical Writer At Applied Medical
We help PhDs from every discipline get into many different types of careers, including Regulatory Affairs.
We show you how to leverage the skills you already have to get hired into a myriad of industry jobs, including Regulatory Affairs.
We show you how to get the job, not how to do the job because each company has their own proprietary systems and will teach you how to do the job through on-the-job training.
But, before you can get this training, you have to get hired first.
That’s where we come in.
We will guide you on how to use your PhD to get hired into Regulatory Affairs.
